Imine Understanding
As can be seen from most of the recent changes on the UsefulChem wiki, Khalid, Alicia and James have been uploading a lot of NMRs in JCAMP format. These have mainly been variations of the basic experimental design of closely monitoring the course of the Ugi reaction by stepwise addition of reagents.
There are many advantages to carrying out Open Chemistry in this format. First, any observations and conclusions that we make can be verified in detail by anyone. Second, many unstated assumptions generally part of the traditional publication process in organic chemistry, such as the purity of the starting materials or the ability of a researcher to read a spectrum without error, can be interrogated independently. Third, there is a wealth of information available to be mined for other investigations beyond the scope of our project. For example, the presence and kinetics of side reactions can be explored by other researchers without having to repeat the experiment. This philosophy or re-purposing and mining experimental results is common in bioinformatics but has not yet caught on in chemistry, especially organic chemistry.
Although our analysis is not complete, we have uncovered some important information relating to the formation of the imine, which is the first step of the Ugi reaction:
1) Phenylacetaldehyde does not react cleanly with a primary amine, such as t-butylamine in CDCl3 and presumably methanol as well. This suggest that other benzylic aldehydes, such as DOPAL, may not react cleanly either and this has important consequences for our synthetic strategy. There have been published attempts of the creating of an imine between phenylacetaldehyde and t-butylamine (Verhe 1980) in CCl4. However, closer inspection of that article reveals that the imine was not characterized and used as a crude product in a subsequent step. If NMR monitoring of this reaction had been made available to the community, it would have saved us (and many others working on other projects I am sure) a lot of time.
2) Aromatic aldehydes such as piperonal and veratraldehyde react cleanly, although more slowly, with the primary amine 5-methylfurfurylamine. Furthermore the equilibrium (at least in CDCl3) appears to be shifted sufficiently towards the imine, making removal of the water a non-issue in optimizing this reaction. Thus we will use aromatic aldehydes to complete the characterization of a Ugi reaction by NMR monitoring before attempting to modify conditions for benzylic aldehydes (needed for many of our targets). We also have enough data to report second order kinetic rates, which we will report on shortly in the relevant experiments.
Although the use of JSpecView is considerably more convenient than paper (e.g. unlimited peak magnifications), there are still some issues. Firefox tends to crash after intensive use going back and forth between spectra, usually after about 20 times. This is always easily corrected by killing and restarting the browser. Also, it is possible to superimpose spectra using BLOCK files in JSpecView and I will post on this as soon as a final glitch has been worked out (the reference position is ignored and spectra appear about 1.6 ppm off). Robert Lancashire has told me he will try to fix this in the coming week and I appreciate his help so far, especially on Christmas day :)
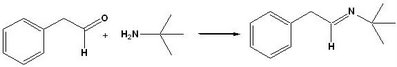




0 Comments:
Post a Comment
<< Home